
【Product Name】
COVID-19 IgM/IgG Ab Test
【INTENDED USE】
COVID-19 IgM/IgG Ab Test is used for qualitative detection of the IgM and IgG antibodies of COVID-19 in human serum/plasma or whole blood. The COVID-19 IgM/IgG Ab Test is an aid in the diagnosis of patients with suspected COVID-19 infection in conjunction with clinical presentation and the results of other laboratory tests. Results from the COVID-19 IgM/IgG Ab Test should not be used as the sole basis for diagnosis.
IgM : sensitivity -89.4%,specificity – 98.4%,accuracy- 94.8%
IgG : sensitivity -91.4%,specificity – 98.6%,accuracy – 95.7%
IgM &IgG :sensitivity -93.9%,specificity – 98.1%,accuracy – 96.4%
√Fast to read result
√High accuracy
√Convenient to use
【TEST PROCEDURE】
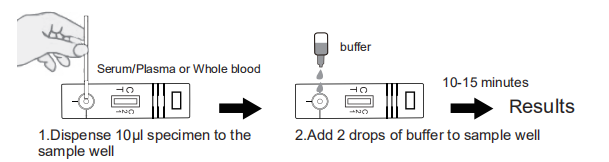
Read the instruction first prior to testing. Bring the pouched test and to room temperature prior to testing. Do not open the pouch until ready to begin testing.
1. Remove the test from the sealed pouch. Lay it on a flat, clean and dry surface.
2. Use the 10μl pipette provided to withdraw the specimen to the marked line. Dispense the specimen to the sample well.
3. Hold the buffer bottle vertically and add 2 drops of buffer (approximate 70μl)to sample well.
4. Read results in 10-15 minutes.
【INTERPRETATION OF RESULT】
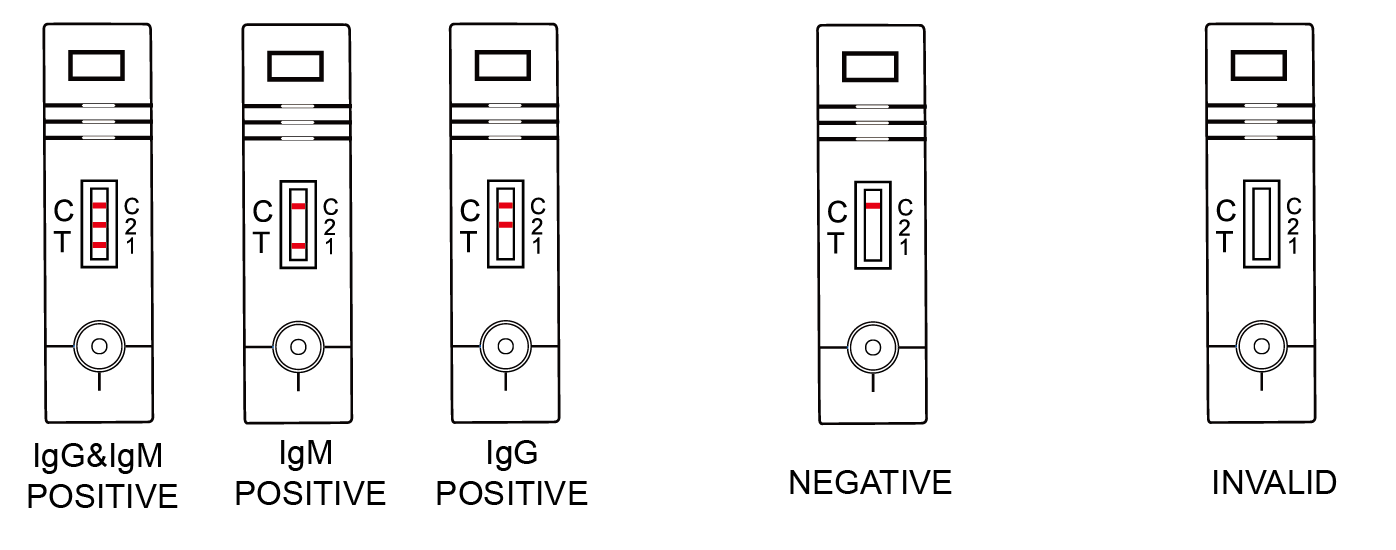
IgG&IgM Positive: Control line and T1 line & T2 line appear in the test Window.
IgM Positive: Two colored lines appear, one is in T1 area and the other line is in Control area.
IgG Positive: Two colored lines appear, one is in T2 area and the other line is in Control area.
Negative: Only one line appears in Control Area, No line appears in T1/T2 area.
Invalid: If no line appears in the Control area, the test results are invalid regardless of the presence or absence of line in the test area. The direction may not been followed correctly or the test may be deteriorated. It is recommended that repeat the test using a new device. If the problem persist, please stop to use the product and contact local distributor.
【INTERNATIONAL MARKETING】
Our COVID-19 IgM/IgG Ab Tests have been exported to 17 countries: Hungary, Indonesia, Slovakia, Ukraine, Uzbekistan, Poland, Italy, Pakistan, Lithuania, Germany, Peru, Morocco, Mongolia, Albania, Romania, Greece, Czech Republic. The COVID-19 IgM/IgG Ab Test is exported and sold to foreign countries only.
This test has not been authorized for use by the FDA and is not available in the United States.